Single-use endoscopic linear stapler and cutting components with five rows of staples.
Classification:
Key words:
Single-use endoscopic linear stapler and cutting components with five rows of staples.
 Product Consulting
Product Consulting
Product Details
【Product Name】Disposable five-row nail linear cutting stapler and cutting component for endoscope
【Product Model Specification】Stapler: WNHM
Cutting components: WNHZ-45A1, WNHZ-45B1, WNHZ-45E1, WNHZ-45F1, WNHZ-45G1
WNHZ-45A2, WNHZ-45B2, WNHZ-45E2, WNHZ-45F2, WNHZ-45G2
WNHZ-60A1, WNHZ-60B1, WNHZ-60E1, WNHZ-60F1, WNHZ-60G1
WNHZ-60A2, WNHZ-60B2, WNHZ-60E2, WNHZ-60F2, WNHZ-60G2
【Performance Indicators】
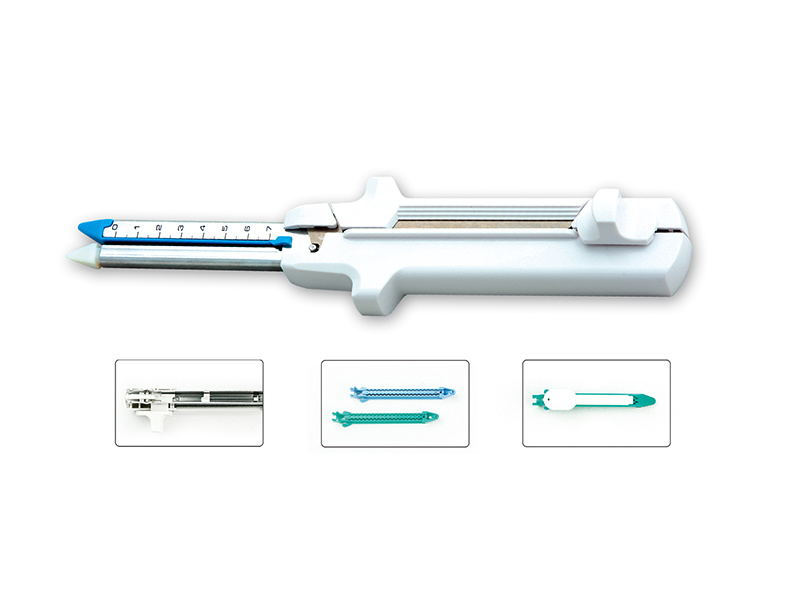
1. Performance: The disposable five-row nail linear cutting stapler and cutting components have a smooth appearance, clear contours, and no defects such as burrs, scratches, or rust. The stapler should open and close flexibly without any jamming. The reset pull knob should fully reset when pulled back to the bottom. The stapler should have good stapling and cutting performance, with neat cutting edges and no burrs after each stapling. The stapled joint should withstand a pressure of no less than 3.6 kPa, with no more than 10 drops of leakage within 15 seconds. The cutting blade should be sharp, and the cutting force should not exceed 0.80N. The stapler has a safety protection device for the empty nail chamber. The nail chamber component should be securely connected to the body and withstand a pulling force of 30N without loosening. 1. Center rod 2. Metal outer sleeve 3. Steering knob 4. Steering handle 5. Safety button
6. Reset pull knob 7. Unloading button 8. Movable handle 9. Fixed handle
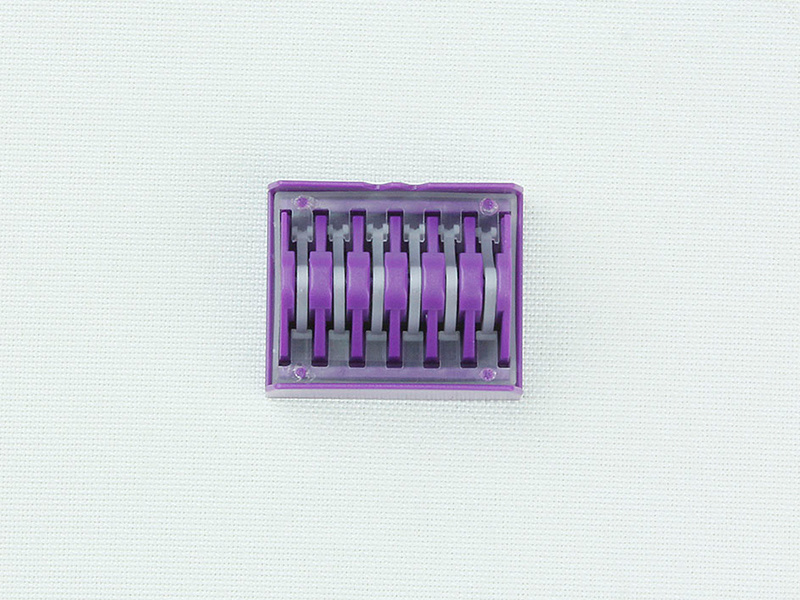
1. Nail seat 2. Nail chamber 3. Nail chamber bracket 4. Cutting knife 5. Staple
3. Materials: The nail seat, nail chamber bracket, and cutting knife are made of 20Cr13 material; the fixed handle, steering handle, and steering knob are made of ABS material; the reset pull knob is made of PC material; the movable handle, safety button, and nail chamber are made of PA66 material; the staples are made of TA2G material.
4. Dimensions:
Table 1 Cutting component internal staple height description Unit: mm
| Intraoperative cutting component Staple height code |
Nail height |
Limit Deviation |
||
| Inner row |
Middle row |
Outer row |
||
| A |
4.8 |
4.8 |
4.8 |
±0.2 |
| B |
3.5 |
3.5 |
3.5 |
|
| E |
4.2 |
4.2 |
4.2 |
|
| F |
4.0 |
4.5 |
5.0 |
|
| G |
3.0 |
3.5 |
4.0 |
|
Table 2 Basic dimensions of intraoperative cutting stapler body Unit: mm
| Model specification of the body |
Length of metal outer sleeve (L1) |
|
| Basic dimensions |
Limit deviation |
|
| WNHM |
155 |
±5.0 |
Table 3 Basic dimensions of intraoperative cutting stapler cutting component Unit: mm
| Model specification |
Stapling length (L) |
Bending angle (W/°) |
Number of nails |
||
| Basic dimensions |
Limit deviation |
Basic dimensions |
Limit deviation |
(pieces) |
|
| WNHZ-45(A,B,E,F,G)(1,2) |
44.5 |
±2.0 |
37 |
±10 |
55 |
| WNHZ-60(A,B,E,F,G)(1,2) |
60.5 |
37 |
±10 |
75 |
|
【Scope of Application】
Applicable for tissue resection, transection, and anastomosis in laparoscopic surgeries in abdominal surgery, gynecology, pediatrics, and thoracic surgery, not for side-to-side anastomosis.
【Usage Method】
Open the sterile packaging of the stapler or the body and components to take out the stapler body and components (do not remove the yellow protective cover on the component at this time), align the “↑” on the head of the stapler and the tail of the component, press down and rotate clockwise. A “click” sound indicates that the body and component are connected properly.
Note 1: If the component cannot be assembled smoothly with the body, please ensure that the black reset pull knob on the body has been pulled to the bottom, or check that the steering knob on the dial is parallel to the welding line on the body.
Note 2: When assembling the component and body, do not remove the yellow protective cover on the component in advance, otherwise the following consequences may occur: the component and body can be assembled normally, and cutting and suturing can be performed normally during the operation; however, after suturing, the cutting knife cannot reset, and the head of the component cannot open, resulting in the inability to remove the tissue.
Remove the yellow protective cover from the component, grip the movable handle to close the head of the component, and use the puncture device to reach the desired surgical site.
Note 1: If after connecting the component and body, gripping the movable handle does not allow the head of the component to close smoothly, it indicates that the component and body are not connected properly. Please refer to the method in step 6 to remove the component and reassemble it. Forcing it at this time may break the internal rack of the gun body, leading to equipment scrapping.
Note 2: After connecting the component to the body of the instrument, be sure to confirm that the "two rows of nails" text marking on the component is located on the "left" or "right" side of the body’s directional knob, to facilitate determining that the pre-excised tissue and the "two rows of nails" text marking are on the same side during surgery. (When excising diseased tissue, the component is inside the body and may not be easily visible to the doctor.)
Pull back the black reset pull knob to open the head of the component (the component is rotatable and can be adjusted by turning the directional knob on the stapler as needed for the surgery), allowing the tissue to be excised to be smoothly placed between the nail chamber and the anvil, adjusting the tissue to be cut so that it is evenly spread and ensuring that no excess tissue is pinched.
Note: When placing tissue into the jaws of the component, pay attention to the "two rows of nails" text marking on the component, rotate the handle to ensure that the pre-excised tissue and the "two rows of nails" text marking are on the same side.
4. After gripping the movable handle to clamp the tissue, release the movable handle, press the green safety button, and begin the surgical operation. Each time the movable handle is squeezed, it will advance and cut 15mm. (After pressing the green safety button to start cutting, it must be cut all the way through; do not pull the black reset pull knob midway.) The specific position for the stapling cut can be referenced by the black scale line next to the black reset pull knob on the body of the instrument (or the black scale line at the bottom of the component).
Note: Remember to press the green safety button before firing; otherwise, it may cause the internal rack of the instrument to break, leading to the instrument being scrapped.
5. After the stapling and cutting process is complete, pull back the black reset pull knob on the body of the instrument to release the clamped surgical tissue. Grip the movable handle once more, and after the head of the component closes, withdraw the instrument.
Note: After cutting is complete, pull back the black reset pull knob, and do not grip the movable handle; otherwise, the black reset pull knob cannot be pulled back.
Method for replacing the component: If a component needs to be replaced during the same surgery, first pull back the black reset pull knob to open the head of the component, then pull back the blue release button on the dial, and turn the component counterclockwise to remove it. Repeat the operation in step 1 to install a new component.
After the stapling and cutting is complete, remove the excised tissue, cut a certain width of undamaged tissue between the suture line and the cutting edge, and send it for pathological examination.
Component configuration: The cutting stapler body can be matched with various cutting components, and the cutting components are single-use products.
[Precautions]
This product must strictly follow sterile operation specifications during use.
Doctors should carefully read this instruction manual before use.
This product is intended for use by physicians who have received professional training or under the guidance of physicians with relevant experience.
Before use, confirm that the component matches the body to be used and select a suitable model of the puncture device. The sizes of minimally invasive laparoscopic staplers may vary between different manufacturers. If different manufacturers' minimally invasive laparoscopic surgical instruments and their accessories are used in the same surgery, compatibility must be verified before the operation.
Preoperative radiotherapy may cause changes in tissue. For example, these changes may cause tissue thickening that exceeds the specified range of the selected stapler. Any preoperative treatment for the patient should be carefully considered, and it may be necessary to change the surgical technique or method.
Before use, check whether the blister packaging is damaged; damaged products should not be used.
The maximum firing count for the instrument body is 5 times; a single instrument body can have its component replaced a maximum of 5 times during the same surgery.
Do not exceed the maximum firing count for component replacement. Using the stapler with reinforcing materials may reduce the firing count.
Before using the component, ensure that the protective cover is intact; otherwise, check whether there are missing staples inside the component; otherwise, the stapler may experience loading failures.
After withdrawing the instrument, be sure to check whether there is any bleeding from the sutured tissue. If there is oozing, cauterization or a few stitches must be applied to the corresponding area.
Ensure that the tissue thickness is within the specified range and that the tissue is evenly distributed within the stapler. Excessive tissue on one side may lead to poor stapling and may cause leakage at the anastomosis.
Selection of stapler height reference is as follows:
◆ Type A nail chamber (stapler height 4.8mm) cannot be used for tissue with a compressed thickness of less than 2.5mm. Or it cannot be appropriately compressed to a thickness of 2.8mm for tissue and the aorta.
◆ Type B nail chamber (stapler height 3.5mm) cannot be used for tissue with a compressed thickness of less than 1.5mm. Or it cannot be appropriately compressed to a thickness of 2.0mm for tissue and the aorta.
◆ Type E nail chamber (stapler height 4.2mm) cannot be used for tissue with a compressed thickness of less than 2.0mm. Or it cannot be appropriately compressed to a thickness of 2.5mm for tissue and the aorta.
◆ Type F nail chamber (stapler height 4.0/4.5/5.0mm) cannot be used for tissue with a compressed thickness of less than 2.25mm. Or it cannot be appropriately compressed to a thickness of 3.0mm for tissue and the aorta.
◆ Type G nail chamber (stapler height 3.0/3.5/4.0mm) cannot be used for tissue with a compressed thickness of less than 1.5mm. Or it cannot be appropriately compressed to a thickness of 2.25mm for tissue and the aorta.
In cases of excessive or overly thick tissue, attempting to forcefully fire the stapler may result in incomplete sutures, potentially causing the anastomosis to rupture or leak. Additionally, instrument damage or firing failure may occur.
Firing must be completed in one go. Do not partially fire the instrument. Incomplete firing may lead to abnormal formation of staples, incomplete cutting lines, bleeding and leakage from the suture line, and/or difficulty in removing the instrument.
During abdominal surgery, if a suitable pneumoperitoneum cannot be formed and maintained, it may reduce the available working space and increase the risk of injury to the visceral tissue.
MRI magnetic resonance compatibility and limited use conditions: Static magnetic field strength less than or equal to 3T, spatial magnetic field gradient not exceeding 720-Gauss/cm. During a 15-minute MRI scan, the maximum whole-body average specific absorption rate (SAR) does not exceed 3.0W/kg. In a 1.5T magnetic field environment, the maximum temperature rise due to RF heating of the anastomosis staples does not exceed 1.6℃; in a 3T magnetic field environment, the maximum temperature rise does not exceed 1.9℃.
This product is sterilized packaging and is limited to use by the same person in the same surgery. Use by multiple patients may jeopardize the integrity of the instrument or pose a risk of contamination, which may lead to patient harm.
This product is sterilized using ethylene oxide (EO), with a validity period of three years from the date of sterilization. Use is prohibited if the inner packaging is damaged or past the expiration date; production date and expiration date are indicated on the certificate of conformity.
This product is strictly prohibited from being re-sterilized;
Please dispose of according to regulations after use;
This manual cannot be used as a basis for clinical surgical anastomosis technique guidance. For questions regarding clinical surgical anastomosis technique guidance, please refer to professional literature or consult this company and its agents.
[Contraindications]
Contraindicated for severe mucosal edema.
Contraindicated for the aorta.
If proximal or distal preventive measures are not provided, do not use the endoscopic cutting anastomosis device on large blood vessels.
Contraindicated for ischemic or necrotic tissue.
Contraindicated for liver or spleen tissue. This is due to the compressive characteristics of such tissues, which may cause destructive effects during closure of the instrument.
Contraindicated for areas where hemostasis cannot be observed.
Cannot be used in situations where anastomosis staples are prohibited.
Cannot be used for side-to-side anastomosis surgery.
Not suitable for surgeries that do not require tissue sampling biopsy.
Before firing the anastomosis device, assess the tissue thickness. If the tissue cannot be easily compressed to the closure height of the anastomosis staples or is too easily compressed to that height, then this tissue is not suitable for this anastomosis staple (it may be too thick or too thin).
[Product Packaging]
1. When the product leaves the factory, the inner packaging is a sterile blister box, and the outer packaging is a paper box, with one device per box, including a product certificate and user manual. A factory inspection report is included with the shipment; do not use if past the expiration date or if the packaging is damaged;
Warning: Do not re-sterilize. If the packaging is damaged, it has been sterilized with ethylene oxide. Sterile. Refer to the user manual.
Use: Never use.
[Product Storage]
Store in an indoor environment with a relative humidity not exceeding 80%, free from corrosive gases and well-ventilated. Ensure that the text and symbols on the packaging remain clear and legible. Prevent impact and compression, and place in a ventilated, dry environment free from corrosive gases.
[Manufacturer]
Manufacturer/registrant/after-sales service unit: Changzhou Weikai Medical Technology Co., Ltd.
Address: No. 9 Changyang Road, Wujin Economic Development Zone, Jiangsu.
Production address: No. 9 Changyang Road, Wujin Economic Development Zone, Jiangsu (West side of Building C4, Xitaihu Medical Industry Incubation Park, second, third, and fifth floors).
The tissue positioning button ensures the correct cutting suture length.
A complete range of specifications suitable for various surgeries.
The same instrument can be replaced to accommodate different tissue thicknesses.
The same instrument can be replaced with different colored staple cartridges to suit different thicknesses.
Recommend Products
Committed to R & D, production and sales of surgical, urological anorectal surgery, minimally invasive surgical instruments and medical supplies
Online Message
If you have any suggestions, please leave a message or send an email to us, and we will reply to you within 1 working day after receiving the message email.
Address: No.9 Changyang Road, Wujin Economic Development Zone, Changzhou, Jiangsu
Mobile: +86-18915067665
E-mail: czwecare@163.com
Quick Navigation

Mobile website QR code