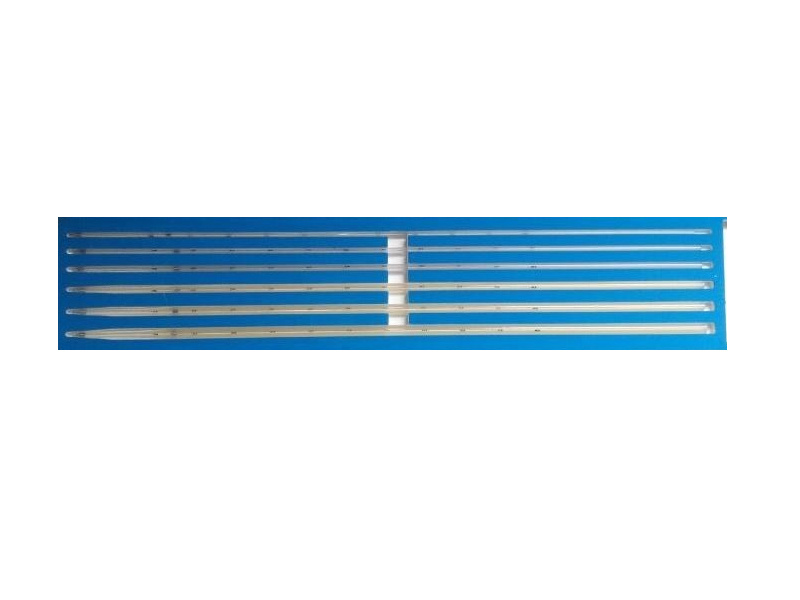
Esophageal cardia stricture dilator
Classification:
Key words:
Esophageal cardia stricture dilator
 Product Consulting
Product Consulting
Product Details
Instructions for Use of Esophageal Cardia Stricture Dilator
[Product Name]
Esophageal Cardia Stricture Dilator
[Model Specifications]
Divided into six specifications based on the diameter of the dilator strips: 5, 7, 9, 11, 13, 15. See Table 1.
Table 1 Basic Dimensions Unit in millimeters
Dilator Strip Specifications | L | D | d | L1 | Cone Length L2 | Guide Wire | ||
Diameter | Safety Soft Spring | Total Length | ||||||
5 | 830±10 | 5 | ≥1.2 | 15 | 125±25 | φ0.8 φ1.0 | 20, 30, 40, 50, 60 | 2200 1600 |
7 | 830±10 | 7 | ≥1.2 | 15 | 125±25 | φ0.8 φ1.0 | 20, 30, 40, 50, 60 | 2200 1600 |
9 | 830±10 | 9 | ≥1.2 | 15 | 125±25 | φ0.8 φ1.0 | 20, 30, 40, 50, 60 | 2200 1600 |
11 | 830±10 | 11 | ≥1.2 | 15 | 125±25 | φ0.8 φ1.0 | 20, 30, 40, 50, 60 | 2200 1600 |
13 | 830±10 | 13 | ≥1.2 | 15 | 125±25 | φ0.8 φ1.0 | 20, 30, 40, 50, 60 | 2200 1600 |
15 | 830±10 | 15 | ≥1.2 | 15 | 125±25 | φ0.8 φ1.0 | 20, 30, 40, 50, 60 | 2200 1600 |
Where: D tolerance is not greater than ±10%, L1 tolerance is not greater than ±3, guide wire tolerance is not greater than ±5%.
[Product Performance]
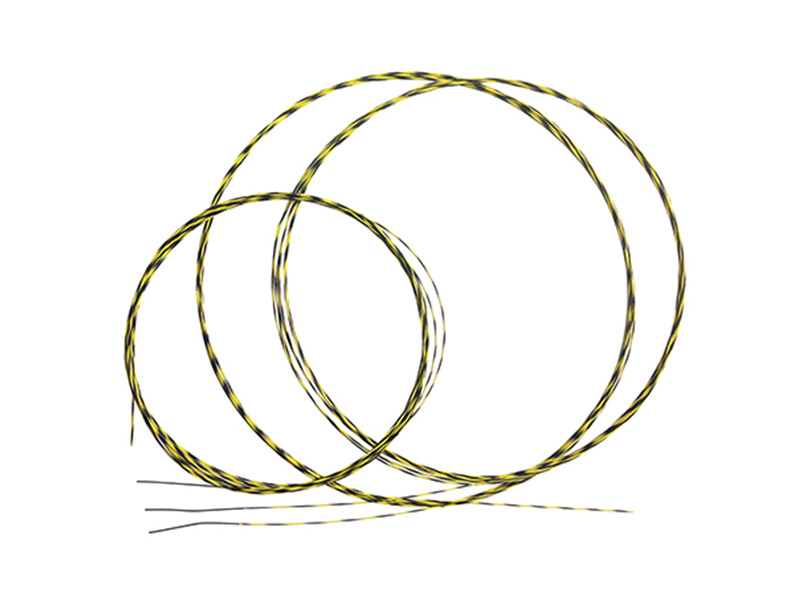
The diameters of the dilator strips are 5, 7, 9, 11, 13, 15mm, available for clinical selection. Guide wire diameters: φ1.0mm, φ0.8, lengths: 1600 mm, 2200mm;
2. The dilator strips have moderate softness, with a smooth cone at the front, which can expand the narrowed area while reducing harm to the body;
3. The dilator strips have X-ray radiopaque markers to determine their position within the body;
4. The dilator is chemically stable, has good biocompatibility, does not cause skin irritation or allergic reactions, and has no acute systemic toxicity.
[Usage Method]
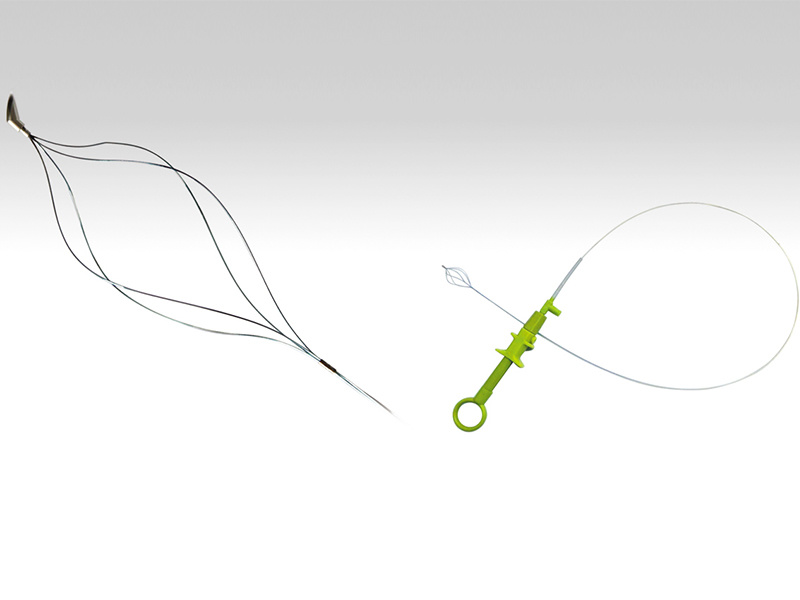
Routine disinfection is required before use. During use, observe the patient's esophagus or cardia narrowing condition via X-ray or endoscopy, select a dilator strip with a diameter slightly larger than the patient's narrowed area, insert the guide wire through the endoscope channel under X-ray monitoring until the soft spring at the front of the guide wire is in the gastric cavity (the shape of the bent soft spring can indicate whether it is in the stomach), withdraw the endoscope while retaining the guide wire, and slide the dilator strip over the guide wire through the patient's narrowed area to achieve dilation. The selection of dilator strip diameters should be from small to large, gradually expanding.
[Shelf Life]
This product is reusable, with an expected usage of 30 times. Use should be stopped if the dilator strip shows cracks, rigidity, or other adverse phenomena. Use should also be stopped if the dilator strip shows wear or permanent deformation.
[Disinfection Method]
The esophageal cardia stricture dilator must be disinfected before use, using 2% glutaraldehyde disinfectant, formalin, or 75% alcohol for general disinfection.
[Precautions]
During the dilation procedure, hold the guide wire relatively still, and use the dilator strip to slide forward along the guide wire. Do not pull the guide wire outward to avoid retracting the safety soft spring head into the patient's gastric cavity. The guide wire is a consumable item and should be carefully checked before each surgery. If the spring head is bent or deformed, the connection between the spring and the wire is not secure, or if there is rust or unevenness on the wire surface, it should be replaced immediately to avoid accidents. For any suspicious situations or unknown reasons regarding the product, stop using it immediately and notify the manufacturer.
[Scope of Application] For dilation treatment of esophageal and cardia strictures.
[Contraindications]
Esophageal stricture deformities and curves caused by mediastinal tumors reaching 60°;
Severe esophageal varices;
Accompanied by aortic aneurysm or heart and lung failure;
Within three months post esophageal or cardia tumor surgery;
Those with coagulation mechanism abnormalities and contraindications for endoscopy.
[Production Date, Expiration Date] See the certificate of conformity.
[Product Packaging]
1. Hard box packaging, each box contains one set of esophageal cardia stricture dilator, along with a finished product inspection report and product instructions;
2. Each set of products has labels on the hard box printed with the following content:
(1) Product name, model, specifications;
(2) Company's name, address, contact information;
(3) Medical device registration certificate number;
(4) Name, address, production address, and production license number of the manufacturing enterprise;
(5) Production date.
The tissue positioning button ensures the correct cutting suture length.A complete range of specifications suitable for various surgeries.
The same instrument can be replaced to accommodate different tissue thicknesses.
The same instrument can be replaced with different colored staple cartridges to suit different thicknesses.
Recommend Products
Committed to R & D, production and sales of surgical, urological anorectal surgery, minimally invasive surgical instruments and medical supplies
Online Message
If you have any suggestions, please leave a message or send an email to us, and we will reply to you within 1 working day after receiving the message email.
Address: No.9 Changyang Road, Wujin Economic Development Zone, Changzhou, Jiangsu
Mobile: +86-18915067665
E-mail: czwecare@163.com
Quick Navigation

Mobile website QR code